Rescuing the Ozone Layer
- Joanne Lee
- Mar 10, 2022
- 2 min read
By: Jeet Parikh

Many of you may remember hearing about the massive hole in the ozone layer over Australia and Antarctica. What happened to it? Is it gone? Not quite. Before we get into this, however, let's understand what the ozone layer is. Ozone is a common gas found in our atmosphere. It is made of three oxygen atoms and is mainly located in the stratosphere. Ozone is extremely important to life on earth, as it absorbs harmful UV-B radiation from the sun.
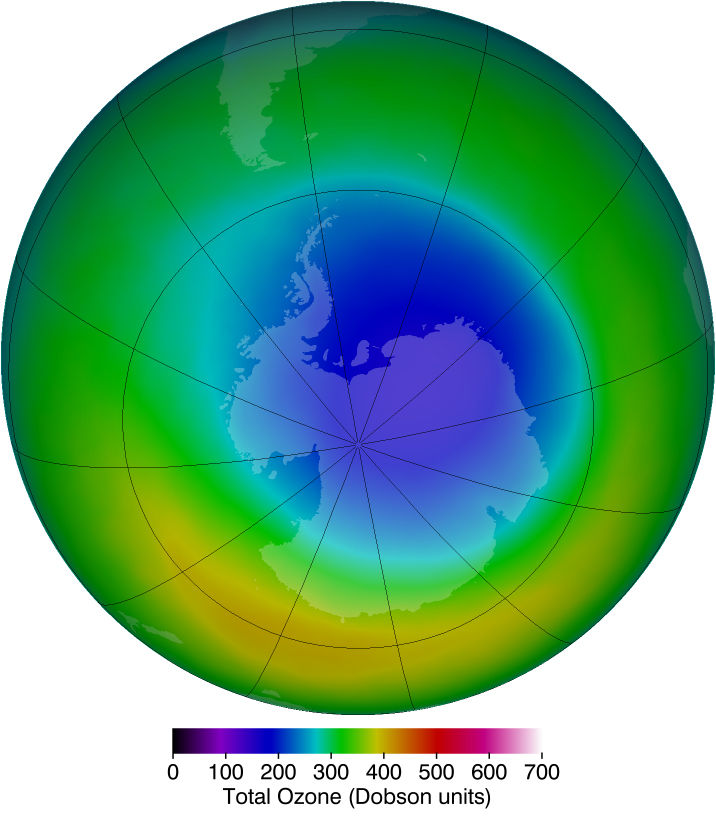
Despite this knowledge, in the late 19th century, scientists noticed something was amiss. Chlorofluorocarbons (CFCs), a new chemical for refrigeration, were being mass-produced around the world for their unique, supposedly non-toxic properties. These chemicals were indeed non-toxic to humans, but in the atmosphere, they had disastrous impacts. In a process called photodissociation, UV radiation could break the chlorine off of the CFC molecule. This detached, or free chlorine atom would steal an oxygen atom for ozone, forming chlorine monoxide (ClO) and oxygen gas (O2). Then, the chlorine in ClO detaches from the molecule, again due to photodissociation, which leads to a vicious cycle of ozone destruction.
Later, in 1984, scientists found a massive hole in the ozone layer, and further research revealed the driving force of CFCs in this depletion; The Montreal Protocol was signed with the goal of halting CFC production. Without added CFC emissions, the cycle of ozone depletion would eventually stop. This logic fundamentally makes sense, so one might wonder why in 2015, decades after the Montreal Protocol, the ozone hole reached an all-time high. Here, it is important to point out that CFCs are not the only source of ozone depletion–bromine gas from volcanic eruptions has a similar effect. Volcanic eruptions will always disrupt the ozone layer, but the policies targeting CFCs have helped preserve the layer. Scientists believe that the ozone layer will be back to pre-1980 levels in around 50 years. So while the ozone hole is not gone, it is shrinking, and on the path of recovery.
Citations:
Images:
What Did You Learn?
Questions:
What is the ozone layer?
The ozone layer is made up of a colorless gas called ozone, which is composed of three oxygen molecules bound together. The ozone primarily resides in the stratosphere, a layer of the atmosphere. In the 1980s, scientists discovered a massive hole in the ozone layer over Australia and Antarctica.
How do chlorofluorocarbons (CFCs) cause ozone depletion?
CFCs are special molecules developed in the 1980s for use in refrigeration mechanisms. When CFCs get into the atmosphere, the Chlorine atom breaks off due to a process called photodissociation. This free chlorine atom binds to oxygen in the ozone molecule, effectively stealing it to form a different compound. Ozone turns into normal oxygen gas, and chlorine binds with the oxygen to form chlorine monoxide.




Comments