Enzymes
- Joanne Lee
- Mar 21, 2022
- 3 min read
By Andrew Gao
Enzymes are molecules that act as biological catalysts. Biological catalysts speed up the rates of chemical reactions, causing the reactions to happen more. They do this by lowering the activation energies of reactions. The activation energy is the minimum energy to trigger a reaction to start. For example, the lowest volume that your alarm has to be to get you up in the morning would be your activation energy. Enzymes are very important in biological processes.
Some enzymes are completely made up of proteins but some have parts made of cofactors. Cofactors are known as "helper molecules" and can come in the form of metal ions like magnesium.
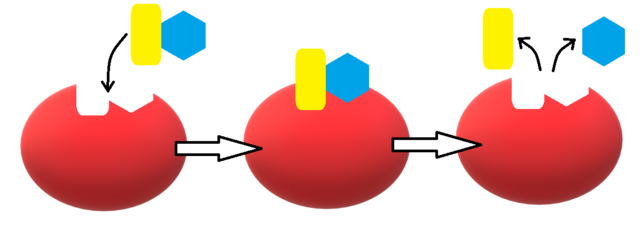
Enzymes are generally specific in their target, known as the substrate. A substrate is what enzymes target and latch on to. At a basic level, enzymes attach themselves to a region on the substrate and then magic happens.
The part of the enzyme that actually ends up "touching" the substrate is known as the active site. When the substrate "binds", or connects, to the active site, reactions can occur. Enzymes often turn substrates into a bunch of new molecules, called products.
Enzymes can speed up rates of reactions by crazy levels! For example, orotidine 5'phosphate decarboxylase can reduce a reaction time from millions of years to milliseconds!
So how exactly do enzymes work? Well, when they bind to substrates, they hold them in a specific way, making it easier for chemical bonds to break and form.
Sometimes, enzymes split up a substrate into two parts. In other cases, they can combine two substrates into one part. There are many other types. In some cases, enzymes speed up reactions by collecting and bringing two molecules together so the two molecules can react. Enzymes can also bend substrates so that it's easier to break the molecular bonds and progress through the reaction.
Also, how do enzymes know to only target specific molecules? Great question. Enzymes have very specific structures, caused by the amino acids comprising it, each with different properties. The shape and charge of an enzyme only allow it to bind to specific molecules that have matching properties at the site of binding. For example, think of a lock and key. The shape of the key matches the grooves inside the lock. However, that key would not be able to fit inside a different lock.
While the lock and key model is very helpful to visualize enzymes and substrates, it's not perfect. When an enzyme connects with a substrate, due to chemical reactions between the two, the shape of the enzyme will change and it will bind even more tightly to the substrate. This phenomenon is called induced fit.
Many factors can change the function of enzymes. High temperatures are well known for speeding up reactions, but having too high temperatures could cause enzymes to denature (basically fall apart and lose its properties). Speaking of temperature, one famous enzyme, Taq polymerase, is useful because of its hardiness at high temperatures. Extracted from T. aquaticus, a bacteria that live in hydrothermal vents, Taq polymerase works well at high temperatures, making it perfect for use in PCR, polymerase chain reaction. This way, PCR can enjoy the effect of high temperature speeding up chemical reactions and not have to worry about the polymerase denaturing. By the way, PCR is used to multiply pieces of DNA.
pH also affects enzymes. The active sites on enzymes commonly are acidic and basic and different pHs can affect the active sites, making it difficult to bind with substrates. pH values that are too high or low can also denature enzymes.
One really interesting thing about enzymes is that they aren't affected at all after participating in a reaction. An enzyme can work for an indefinite amount of times. Once a reaction is finished, the enzyme is once again free and able to bind another substrate.
There are six types of enzymes:
lyases
hydrolases
isomerases
ligases/synthases
oxidoreductases
transferases
I'm not going to explain each of them but you can search them up.
Enzyme activity can be measured in the lab via enzyme assays. This consists of calculating the rate of disappearance of substrates or the rate of appearance of products. This makes sense: the faster substrates disappear, the faster the enzyme works.
Image Credit:
No changes were made, File:Enzymes Lock and Key.png - Wikimedia Commons, License: Creative Commons — Attribution-ShareAlike 4.0 International — CC BY-SA 4.0




Comments